USA ship Mobile Trolly Cart Monitor Stand Roll Wheel Mount For Patient Monitor



We are facotry, any product can give you 10% discount. Please contact me Whatsapp:+8615243475232, conteccare AT gmail com.
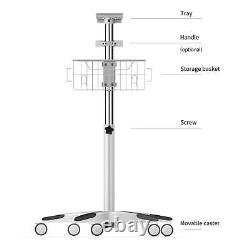
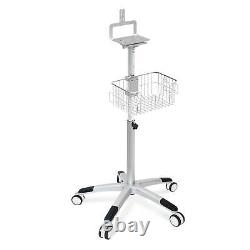
This Trolley/stand/cart is used on CONTEC ICU Patient Monitor. By China : Generally, about 2-3 weeks.
Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe.
The Fingertip Pulse Oximeter that is FDA 510K Approved. 100% satisfaction is our goal! Contec Medical Systems focusing on research, manufacture and distribution of medical instruments, was founded in 1992 as a high-tech company. We have us stock in IL, United States. We are looking forward to establishing a successful business relationship with you.Welcome to be our agent. Only english user guide, if you need any other language, please contact me. This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Patient Monitors". The seller is "contecstore" and is located in this country: US. This item can be shipped to United States.
- Brand: CON-TEC
- MPN: 69450401
- Monitor Parameters: cart
- Intended Use/Discipline: Anesthesiology, Biological Laboratory, Cardiology
- Technology: Digital
- Model: mobile cart for CONTEC CMS8000
- Country/Region of Manufacture: China
- Bundle Listing: Yes