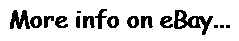Vital Signs Patient Monitor with wall bracket, Stand 7 Parameter CMS8000 USA ship
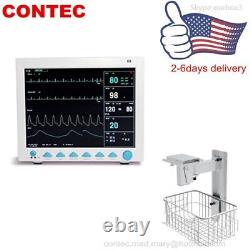
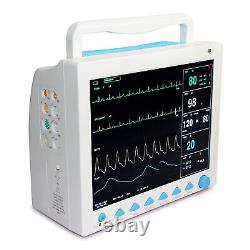
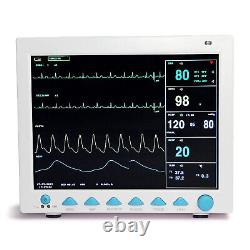







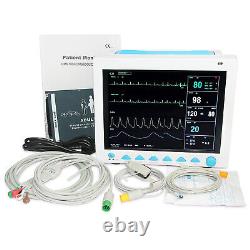

CONTEC FDA&CE ICU CCU Vital Signs Patient Monitor, 6 Parameters CMS8000 with cart. The monitor has abundant functions that can be used for clinical monitoring with adult, pediatric and neonate.
Users may select different parameter configuration according to different requirements. The monitor, power supplied by 100-240V~, 50/60Hz, adopts 12.1'' color TFT LCD displaying real-time date and waveform. It can synchronously display eight-channel waveform and full monitoring parameters equipped with an optional 48mm thermal recorder. The monitor can be connected to the central monitoring system via wire or wireless network to form a network monitoring system. This device can monitor such parameters as ECG, RESP, NIBP, SpO2, and dual-channel TEMP, etc.
It integrates parameter measurement module, display and recorder in one device to form a compact and portable equipment. Its replaceable internal battery brings lot of convenient for patient moving. Standard parameters: ECG, RESP, SpO2, PR, NIBP, dual-channel TEMP. 4NIBP Systolic pressure(SYS), Diastolic pressure(DIA), Mean pressure(MEAN). 6IBP(optional) CH1:SYS, DIA, MAP. It has abundant functions, such as audible and visual alarm, trend data storage and output, NIBP measurement, alarm event marking and drug concentration calculation, etc.112.1'' TFT color LCD, multi-language interface(Simplified Chinese, Traditional Chinese, English, French, German, Turkish, Spanish, Portuguese, Italian, Dutch, Romanian, Russian, Kazakh, Polish, Czech). 2Fanless design, quiet, energy-saving and clean, which reduces the possibility of cross-infection. 3All-round monitor for adult, pediatric and neonate. 4With standard interface, oxygen graph, trend graph, big character interface and view bed, convenient to observe.
5Finish all operations by keys and knobs. 6Maximum 8-channel waveform synchronous display. 7Display 7-lead ECG waveform on one screen, cascade ECG waveform display. 8Adopt digital SpO2 technology, anti-motion and anti-ambient light interference, and measurement can be performed under the circumstance of weak filling. 9Heart rate variability (HRV) analysis function.
10NIBP measurement mode: Manual/AUTO/STAT, storage for 4800-group NIBP data. 11Review for 71 alarm events of all parameters and 60 arrhythmia alarm events. 12Drug concentration calculation and titration table functions.
13One-touch printing of trend graph. 14Connect to Central Monitoring System by 3G, Wi-Fi or wired mode. 15AC/DC, built-in rechargeable lithium battery achieve uninterrupted monitoring. 16Anti-high frequency surgical unit, defibrillation-proof (special leads are necessary). Lead mode: 3-lead or 5-lead. Lead selection: I, II, III, aVR, aVL, aVF, V.Gain: 2.5mm/mV, 5.0mm/mV, 10mm/mV, 20mm/mV, 40mm/mV. Scan speed: 12.5mm/s, 25 mm/s, 50 mm/s. Measurement and alarm range: 15~350 bpm.
Accuracy: ±1 % or ±1 bpm, whichever is greater. Alarm accuracy: ± 2 bpm. Measurement and alarm range: -2.0 mV ~ +2.0 mV. Accuracy: -0.8 mV~+0.8 mV ±0.04 mV or ±10%, whichever is greater. Arrhythmia analysis: ASYSTOLE, VFIB/VTAC, COUPLET, BIGEMINY, TRIGEMINY, R ON T, VT>2.
PVC, TACHY, BRADY, MISSED BEATS, PNP, PNC. Measurement and alarm range: 0~150 rpm. Scan speed: 6.25 mm/s, 12.5 mm/s, 25 mm/s. Measurement interval in AUTO mode: 1/2/3/4/5/10/15/30/60/90/120/240/480/960 minutes. Measurement period in STAT mode: 5 minutes.Measurement and alarm range: 10 ~ 270 mmHg. Cuff pressure accuracy: ±3 mmHg. Maximal mean deviation: ±5 mmHg. Maximal standard deviation: 8 mmHg. Measurement and alarm range: 0 ~ 100%.
Measurement and alarm range: 30 ~ 250 bpm. Measurement accuracy: ±2 bpm or ±2%, whichever is greater. Measurement and alarm range: 0 ~ 50? 0~69 mmHg, 0.1 mmHg. 70~150 mmHg, 0.25 mmHg. 0~40 mm Hg ±2 mm Hg. Label: ART, PA, CVP, RAP, LAP, ICP, P1, P2. Measuring and Alarm Range: -10~300 mmHg.Accuracy: ±2% or 1mmHg, whichever is greater. Safety classification: Class I, type CF defibrillation-proof applied part. 1Adult fingertip SpO2 probe (5-pin).
Dimension: 314 mm(L) × 145 mm(W) × 264 mm(H). Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE, TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved. These charges are buyers' responsibility. ECG / ECG holter EEG / EEG Holter B-ultrasound.
Patient Monitor Fetal doppler Fetal Monitor. Spo2 Monitor Stethoscope Medical Image... We are look for partners in the world now! Do you want to become our agent in you conutry?